Cyclosporine is a widely used, strong immunosuppressant in various fields of organ transplantation and is effective in prolonging the survival of transplanted organs. The use of this drug usually follows a fixed application rule: the initial dose is 15 mg/kg, which is then gradually reduced to 5 mg/kg. During this process, it is important to strictly monitor the concentration level of cyclosporine in the body in order to prevent the drug's side effects[1]. The blood level of cyclosporine is affected by various factors, including nutritional status, the use of other medications and the mechanistic environment. Of these factors, diarrhea has a particularly significant effect and can easily cause problems for patients, yet it is often overlooked.
Although diarrhea is usually considered a minor inconvenience, it can have a significant impact on patients taking cyclosporine. Diarrhea occurs in 14%-47% of patients treated with cyclosporine. The relationship between diarrhea and cyclosporine levels is complex and involves altered pharmacokinetics and absorption kinetics.
This case report series aims to provide physicians with practical guidance on how to respond to a patient's diarrhea.
A 12-year-old girl underwent a kidney transplant due to angio-globular nephritis. Following the surgery, she was prescribed cyclosporine (15 mg/kg), methylprednisolone (20 mg/kg intravenously) and anti-thymocyte globulin (1.5 mg/kg intravenously) for two to three weeks. Subsequently, the patient reduced the cyclosporine dose to 12 mg/kg/day, and during this period, cyclosporine TDM showed trough concentrations of around 380 ng/ml, reaching 415 ng/ml in week 7, when the patient experienced loose stools. For this reason, the cyclosporine dose was reduced further to 10 mg/kg/day, and the patient was rehydrated with oral rehydration salts (ORS) and intravenous fluids. The cyclosporine concentration increased to 440 ng/ml in the first week and the dose was reduced again to 8 mg/kg/day. The cyclosporine trough concentration then fell back to 340 ng/ml in the tenth week. During the diarrhea, the cyclosporine concentration eventually fell to 202–300 ng/ml (week 20) and the patient was able to continue the treatment.
A 10-year-old boy underwent a kidney transplant due to vascular glomerulonephritis. The postoperative starting therapy consisted of cyclosporine (15 mg/kg), methylprednisolone (20 mg/kg intravenously) and anti-thymocyte globulin (1.5 mg/kg intravenously). To prevent side effects, TDM was initiated at the beginning of treatment. TDM after three weeks revealed a cyclosporine trough concentration of 368 ng/ml. Over the subsequent three to four weeks of treatment, the cyclosporine dose was reduced to 10–12 mg/kg/day and the cyclosporine trough concentration decreased to 290 ng/ml by week seven. However, diarrhea symptoms developed and the patient received rehydration therapy (oral rehydration salts (ORS) and intravenous (IV) fluids). Nevertheless, the cyclosporine trough concentration quickly increased to 460 ng/ml. The rehydration regimen was modified to ORS only and the cyclosporine dose lowered to 8 mg/kg/day. However, the trough concentration of cyclosporine remained high until week 16, when the diarrhea disappeared, rehydration was discontinued and the trough concentration of cyclosporine decreased to 223 ng/ml.
An 11-year-old boy underwent a renal transplant due to tubulointerstitial nephropathy. His initial postoperative therapy consisted of cyclosporine (15 mg/kg), methylprednisolone (20 mg/kg intravenously) and anti-thymocyte globulin (1.5 mg/kg intravenously). TDM revealed a cyclosporine trough concentration of 376 ng/ml at week 3. He was treated with a dose of 8 mg/kg/day for the following 3 weeks. At week 8, the patient developed a fever and diarrhea, for which rehydration therapy (ORS and IV fluids) and metronidazole were initiated. Thereafter, the cyclosporine trough concentration increased to 423 ng/ml. The cyclosporine dose was lowered to 6 mg/kg/day, but this did not result in a significant reduction in the cyclosporine blood concentration. Once the diarrhea had stopped, the patient was switched to a maintenance therapy of 8 mg/kg/day and the cyclosporine blood concentration was maintained within the target trough concentration.
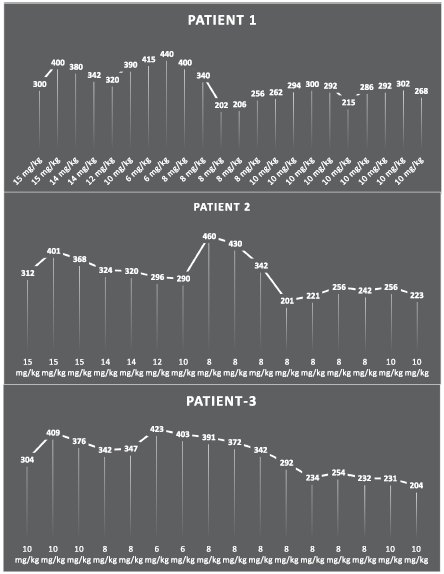
Figure 1. Trend in cyclosporine values through the treatment period. The
figure shows the relation between the various doses of cyclosporine and blood levels. The first peak is normal and indicates the induction phase. The second prominent peak indicates the disproportionate rise in blood levels of cyclosporine following diarrhea.
These three examples demonstrate the effect of diarrhea on cyclosporine blood levels. They inform us that TDM provides a direct basis for physicians to adjust the dosage. However, they also show that diarrhea can cause a significant increase in cyclosporine concentrations and that it is necessary to adjust the dosage to reduce the risk of high concentrations. As there is no established approach to managing diarrhea, drug concentration monitoring is the only available tool. However, as these three cases show, adjusting the dosage based on TDM alone is not effective in reducing blood concentrations; curing the diarrhea is key to recovery. This also emphasizes the need for vigilance regarding disease interactions in addition to TDM testing in the clinic.
Automated Therapeutic Drug Monitoring Platformfor Chemicals and Biologics
Reference:
1. Halloran, P.F., Immunosuppressive drugs for kidney transplantation. N Engl J Med, 2004. 351(26): p. 2715-29.