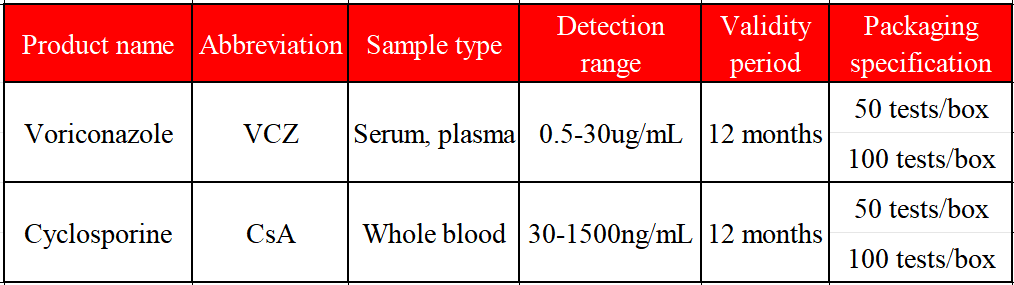
●An 18-year-old female patient after allogeneic peripheral hematopoietic stem cell transplantation took cyclosporine 100 nag once every 12 hours, mycophenolate mofetil 1.0 g once every 12 hours, and methylprednisolone 8 mg once every 12 hours, and the plasma concentration of cyclosporine was maintained at 177-283 ng/L.
● Two months later, voriconazole 200 mg was added orally once every 12 hours due to pulmonary fungal infection. On the 8th day of medication, the patient
Cyclosporine contains a cyclic polypeptide of 11 amino acids and is a commonly used immunosuppressant. This drug is primarily metabolized by the liver. Any drug that inhibits or induces the CYP450 enzyme system can increase or decrease its plasma concentration. Common adverse reactions of cyclosporine include renal damage, hypertension, hirsutism, etc., which may be related to affecting mitochondrial function. It can also cause symptoms of encephalopathy, such as amnesia, disorientation, convulsions, abnormal hearing, blurred vision, etc. In addition, it has been reported that it can also cause Behcet's syndrome, Parkinson's syndrome, etc. Studies have shown that cyclosporine can cause damage to the hippocampus, brain white matter, bilateral lobes, left occipital lobe, left temporal lobe and other parts, and may penetrate the blood and cerebrospinal fluid barrier with a small amount of the drug. These lesions are reversible and can gradually reduce or disappear after reducing the dose, discontinuing or switching to other immunosuppressants [2].
It has been reported that in stable renal transplant patients, voriconazole can increase the maximum plasma concentration and the area under the drug-time curve of cyclosporine by at least 13% and 70%, respectively. After discontinuing voriconazole, the plasma concentration of cyclosporine still needs to be monitored. After the patient was added to voriconazole, the blood concentration of cyclosporine increased by about 30% compared with the upper limit before treatment, and he also developed headache, blurred vision, twitching of limbs and suspicious white matter lesions, which is really rare. This patient was treated with mycophenolate mofetil. Mycophenolate mofetil has adverse effects on the central nervous system and can also cause progressive multifocal leukoencephalopathy (PML). Therefore, the possibility of neurotoxicity caused by the combined action of two immunosuppressants cannot be ruled out [3].
The Importance of TDM for Drug Interaction Tips
Drug interactions are an important cause of adverse drug reactions. According to literature reports, more than 25% of drug-related hospitalizations are caused by potential and harmful drug interactions. However, clinical research data on potentially harmful drug interactions are limited and need to be accumulated over time. Therefore, while paying attention to the interactions between drugs, timely monitoring of drug concentrations is required to facilitate adjustment of patient dosage.
References
[1] Qu Caihong a; Su Xiangyang b. Voriconazole-induced epileptiform seizures related to elevated cyclosporine blood concentration. Journal of Adverse Drug Reactions. 2010, 12(5): 354-3.
[2] lllsiger s, Janzen N, Lficke T, et a1. Cyclosporine A: impact on mitochondrial function in endothelial cells[J]. Clin Transplant, 2010, Jd 14 [Epub ahead of print] http:// online library . wiley. com/DOl:10. I Il l/j. 1399- 0012.2010.01301. xAccepted May 12.2010.
[3] Rougheed EE, Kalisch LM, Barratt JD. Prevalence of potentially hazardous drug interactions amongst Australian veterans[J]. Br J Clin Pharmacol, 2010, 70(2): 252-257.