Therapeutic Drug Monitoring—Anti-Tumor Drugs
Therapeutic drug monitoring (TDM) refers to the process of clinical drug treatment, while observing the efficacy of the drug, regularly collecting the patient’s body fluids (such as blood, urine, saliva, etc.) and measuring the concentration of the drugs or their metabolites, and exploring the biotransformation process of drugs in the body and the safe range of drug concentrations, and based on pharmacokinetics and pharmacodynamics theory, with the help of advanced analytical technology and computer means, design the optimal dosage plan (drug dosage, administration route, medication interval, etc.) to achieve individualized medication. TDM aims to guide patients in the rational use of medications in order to achieve safe, effective, and economical medication goals.
Most drugs with obvious clinical efficacy or low toxicity do not require TDM. At present, the drugs used for TDM at home and abroad mainly include antibiotics, antiviral, anti-tumor drugs, as well as cardiovascular, neuropsychiatric and immunosuppressive drugs. Anti-tumor drugs have a low therapeutic index and relatively high toxicity, and there are wide individual differences in the absorption, distribution, metabolism and excretion processes in the body. Treatment options for cancer patients need to be continuously improved. There are many types of combination drugs, and the treatment effect is closely related to the patient’s basic physical signs. Therefore, therapeutic drug monitoring is necessary when antitumor drugs are used clinically. This article will review the research progress of therapeutic drug monitoring, the application characteristics of anti-tumor drugs, and the clinical application of therapeutic drug monitoring in anti-tumor drugs.
Application characteristics of anti-tumor drugs
Traditional cytotoxic drugs and molecular targeted drugs are the dominant clinical anti-tumor drug treatments today. However, clinically, the risk of cancer patients receiving drug treatment is still high. This is mainly due to poor efficacy, excessive toxicity and drug resistance of the drugs. The particularity of anti-tumor drugs leads to the necessity of therapeutic drug monitoring.
Basic characteristics of anti-tumor drugs
First of all, anti-tumor drugs have a low therapeutic index and a narrow safety range, and it is easy to cause insufficient dosage or excessive poisoning when taking the drugs. Secondly, blood drug concentration has a strong correlation with drug efficacy and toxicity. The area under the concentration-time curve (AUC) and the steady state plasma concentration (Css) can better reflect the efficacy and toxicity of the drug than the dose. Third, drug efficacy and toxicity should not be directly observed and screened. For example, chemotherapy drugs usually need to be taken for multiple cycles to determine whether they are effective. The neurotoxicity of platinum drugs has an accumulation effect, and some anti-tumor drugs also have carcinogenic effects. Fourth, tumors are serious diseases, and their dosage regimen is closely related to the functional level of the patient’s heart, liver, kidneys and other organs. Its treatment effect will directly determine the patient’s quality of life and even life expectancy. The above characteristics indicate that anti-tumor drugs require close monitoring and timely adjustment of blood drug concentrations. At the same time, individualized medication plans must be formulated based on the patient’s own symptoms. TDM will undoubtedly play an important role.
Interactions between anti-tumor drugs and other drugs
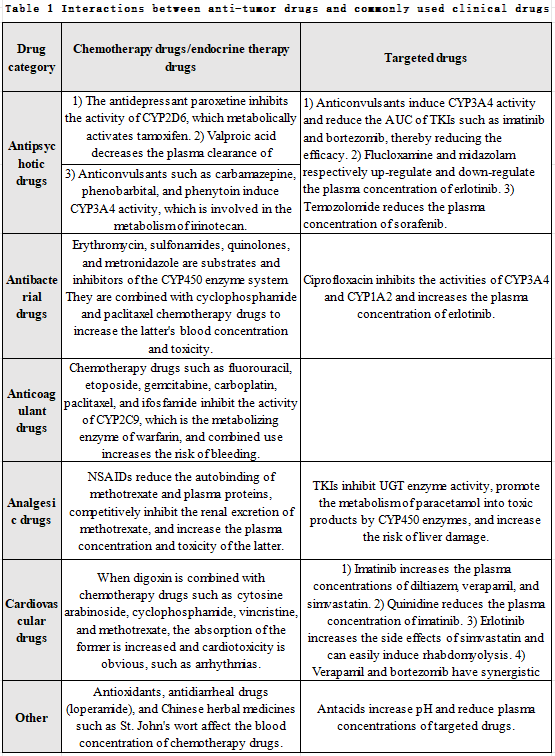
Drug interaction refers to the pharmacokinetic and pharmacodynamic changes that occur when two or more drugs are used simultaneously. In terms of pharmacokinetics, some drugs can regulate the P-glycoprotein (P-gP) and cytochrome P450 enzyme (CYP450) systems, thereby affecting the absorption, distribution, metabolism and excretion of other drugs. In terms of pharmacodynamics, different drugs have additive, synergistic, or antagonistic mechanisms of action, thereby affecting efficacy or toxicity. Cancer patients often take a variety of drugs, mainly because: ① The dosage regimen of anti-tumor drugs often involves a combination of multiple drugs. ② Anti-tumor drugs have serious side effects and need to be adjusted by taking other drugs at the same time. ③ Cancer patients also need to take drugs for other underlying diseases. Studies have found that about 60% of cancer patients have at least one underlying disease and more than one drug interaction risk. Therefore, when formulating a dosing regimen for cancer patients, individual factors such as patient and drug should be fully taken into consideration, and the regimen should be adjusted at any time based on the results of TDM. Cancer patients often also need to take psychiatric, cardiovascular, antibiotic, analgesic and anticoagulant drugs. The interactions between anti-tumor drugs and these drugs are detailed in Table 1.
Clinical application of TDM in anti-tumor drugsThe clinical application of TDM is mainly based on pharmacodynamics and pharmacokinetics research of drugs. Therefore, it is particularly important to find appropriate pharmacokinetic parameter ranges for antitumor drugs in clinical practice. Reports show that the pharmacokinetic and pharmacodynamic relationships of anti-tumor drugs such as methotrexate, fluorouracil, paclitaxel and imatinib are relatively clear, and clinical TDM is relatively mature and can be used to guide clinical medication. At the same time, the TDM of other drugs needs further research.
The therapeutic index of MTX is low, and patients are likely to experience simultaneous drug effects and toxic reactions when taking the drug. When using MTX in large doses, calcium folinate (CF) rescue therapy is required. The CF rescue dose and time should be determined based on the patient’s MTX plasma concentration. Studies have shown that when the plasma concentration of MTX is still higher than 5 μmol·L-1 24 hours after administration or still higher than 0.5 μmol·L-1 48 hours after administration, CF rescue should be given.
It is well known that the activity of dihydropyrimidine dehydrogenase (DPD enzyme) can affect the metabolism of 5-Fu. However, since toxicity cannot be accurately predicted, DPD enzyme can only be used as a reference. Reports show that the toxicity of 5-Fu is closely related to plasma concentration and AUC: after 5 days of administration, if the plasma concentration is greater than 1.5 μmol·L-1 , the patient’s white blood cells will be significantly reduced; after a treatment cycle, when the AUC reaches 30 mg·L-1 ·h-1 , the toxicity of 5-Fu is obvious, and when the AUC reaches 29 mg·L-1 ·h-1, the patient’s life span can be extended. Capecitabine is metabolized into 5-Fu in the body, so the toxicity of capecitabine is also closely related to the plasma concentration.
Paclitaxel is a commonly used drug for the treatment of gynecological tumors. It has been clinically proven that when Taxol is used to treat ovarian cancer, the duration of its plasma concentration greater than 0.05 μmol·L-1 is related to the proportion of tumor complete response (CR) or partial response (PR) and progression-free survival (PFS) is positively correlated; as a common hematological toxicity of Taxol, the percentage of neutropenia is significantly related to the time course of plasma drug concentration greater than 0.1 μmol·L-1; after Taxol combined with epirubicin , the time for the plasma drug concentration to be greater than 0.1 μmol·L-1 will be reduced accordingly, and the toxic and side effects will also be reduced accordingly.
Irinotecan is a camptothecin analogue that exerts anticancer effects after being converted into active SN-38 in the body, but it is also accompanied by severe hematological toxicity. Active SN-38 is converted into inactive SN38G by liver UGT1A1, so UGT1A1 polymorphisms will cause changes in SN-38 levels. During clinical use, toxic and side effects related to CPT-11 can be predicted and discovered in a timely manner by detecting the genotype of UGT1A1 and the level of SN-38.
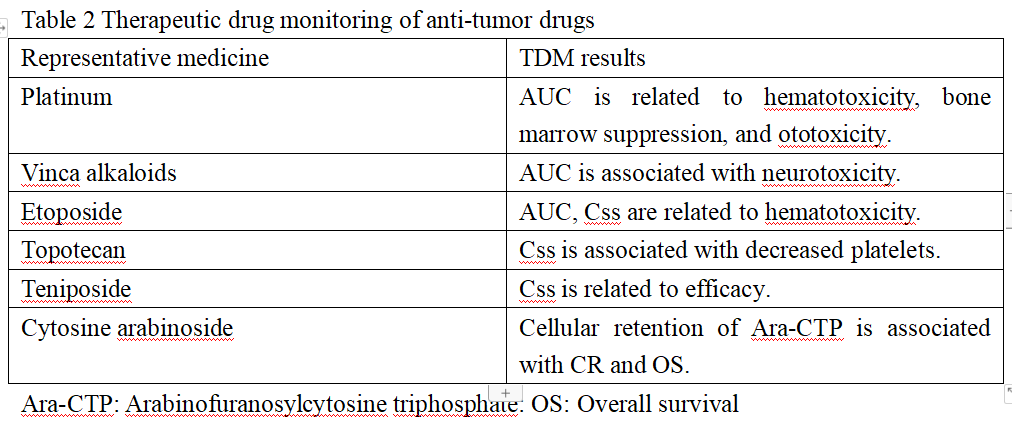
Imatinib is a commonly used targeted drug for the treatment of chronic myelognous leukemia (CML) and malignant gastrointestinal stromal tumor (GIST). Studies have found that the clinical efficacy of imatinib is closely related to its trough concentration. In the treatment of CML, imatinib trough concentration reaching 1170 ng·mL-1 can achieve better therapeutic effect, but the incidence of myalgia, rash, anemia and fluid retention is also higher; in the treatment of GIST, longer PFS can be obtained by imatinib trough concentration reaching 1110 ng·mL-1 . In addition, a series of TDM studies have been conducted on other anti-tumor drugs (see Table 2).
It can be seen that compared with classic pharmacology and molecular biology methods, TDM can judge whether there is treatment failure, poor efficacy or risk of overdose poisoning for patients earlier by promptly observing the patient’s plasma drug concentration (such as plateau concentration, peak concentration, trough concentration, AUC, etc.). A foreign literature studying the treatment of GIST shows that the process for doctors to select targeted drugs for GIST patients is divided into six steps: disease diagnosis, staging, administration, concentration monitoring, efficacy evaluation, and drug adjustment. This is basically consistent with the 7-step process for therapeutic drug monitoring mentioned above.
TDM and rational drug use
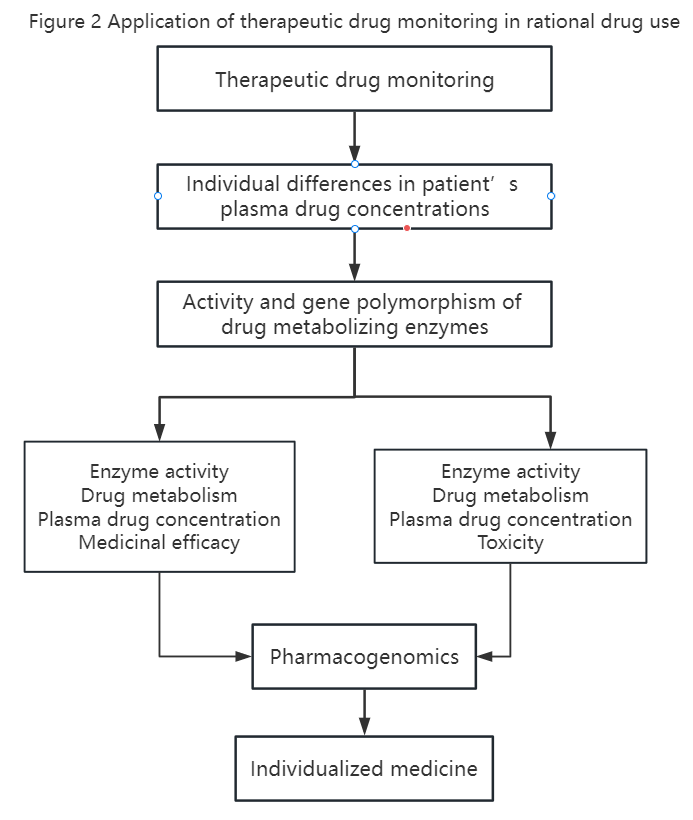
Rational drug use refers to the safe, effective, economical and appropriate use of drugs for patients to achieve the purpose of treating, diagnosing and preventing diseases based on the systematic knowledge and theory of drugs and diseases. According to an announcement issued by the WHO, 50% of patients around the world still fail to use medicines correctly. In our country, the types, dosages, methods and compatibility of drugs used by patients are still serious, resulting in less than expected efficacy and widespread occurrence of adverse reactions and drug resistance. In order to achieve rational drug use, in addition to strictly abiding by rules and regulations and comprehensively grasping drug information, we must also fully consider the individual differences of patients and optimize dosing plans in a timely manner through therapeutic drug monitoring. The types of drugs mentioned above are strong evidence of the rational application of anti-tumor drugs, but clinical practice shows that more extensive research needs to be further carried out. The prerequisite for TDM is a clear dose-effect (toxicity) relationship, that is, establishing the relationship between plasma drug concentration (or AUC, peak and trough concentration) and efficacy and toxicity. TDM can discover individualized differences in patients’ plasma drug concentrations, while pharmacogenomics can predict patients’ efficacy and toxicity. The organic combination of the two is the prerequisite for guiding clinical medication (see Figure 2 for details). However, TDM also encounters some difficulties in practical applications. First, anti-tumor drugs need to be converted into active metabolites in the body to be effective, and active metabolites in the blood are more difficult to monitor than prototype drugs. Secondly, due to abnormal proliferation of blood vessels and special blood supply in tumor tissue, the plasma drug concentration monitored in the systemic circulation may not truly reflect the drug distribution in the tumor tissue. Thirdly, the monitoring period of anti-tumor drugs is long. If the cure rate is used as the efficacy evaluation criterion, or if the cumulative toxicity of the drug is observed, it will take many years of follow-up to obtain complete data. Fourth, cancer treatment often uses combination administration. It is difficult to distinguish the efficacy and toxicity of a single drug during monitoring, and drug interactions must also be considered. Finally, in order to avoid drug toxicity or drug resistance in patients, intermittent administration of anti-tumor drugs is often used in clinical applications. Sometimes it is difficult to achieve steady-state plasma concentration, which makes monitoring difficult.
In summary, due to the particularity of anti-tumor drugs and tumor patients, TDM plays an important role in the process of formulating individualized medication plans, but it also faces severe challenges. At present, domestic TDM research remains more at the theoretical level, with less clinical practice. Only by strengthening theoretical research, continuously deepening clinical practice, accumulating evidence of patient medication, and organically integrating it with pharmacogenomics can we truly "tailor-fit" medication for patients and ensure the rational use of anti-tumor drugs.Diagreat Biotech’s anti-tumor monitoring items1. Blood collection time points: Generally, 24 hours, 48 hours and 72 hours, but the initial blood collection point for the 24-hour dosing regimen is 36 hours after the end of dosing or 42 hours after the start of dosing.
2. Monitoring frequency: The monitoring frequency for patients with normal metabolism is 3 or 4 times; the monitoring frequency for patients with delayed metabolism is based on the plasma concentration of MTX. Monitoring can be stopped until MTX is<0.1umol/L.
1. Blood collection time point: The blood collection time is (24±6) hours after the start of intravenous infusion of 5-fluorouracil, and the start, end and blood collection time of intravenous infusion should be accurately recorded.
2. Monitoring frequency: Monitor once after administration (AUC).
1. Blood collection time point: (24±6) hours after intravenous infusion.
2. Monitoring frequency: Monitor during the infusion of each chemotherapy cycle to facilitate adjustment of the dose for the next cycle.
[1] Li Run'e. Treatment monitoring of anti-tumor drugs[J].Rational Clinical Use of Drugs, 2014, 7(1): 150-151.
[2] Qian Xinhua, Zhang Nengfang, Gao Ling. Treatment monitoring of anti-tumor drugs[J]. Jiangsu Pharmacy and Clinical Research, 2003, 11(6): 36-39.
[3] Lan Jing. Overview and research progress of therapeutic drug monitoring[J]. Tianjin Pharmacy, 2010, 22(3): 53-55.
[4] Wei Xin. Research progress on methodologies for therapeutic drug monitoring[J]. Journal of Anhui Health Vocational and Technical College, 2010, 9(1): 86-87,91.
[5] Xie Jike, Jiang Dechun. Research progress on therapeutic drug monitoring[J]. Chinese Drug Application and Monitoring, 2011, 8(6): 379-382.
[6] GER RITSEN-VAN SCHIEVEEN P, ROYER B. Level of evidence for therapeutic drug monitoring of taxanes [J]. Fundam Clin Pharmacol, 2011, 25(4): 414-424.