How to establish a method for monitoring voriconazole therapeutic drug concentration?
In recent years, the incidence of deep mycoses has been on the rise. Most of the patients are seriously ill and often life-threatening. The pathogens are mainly opportunistic fungi, such as Candida and Aspergillus. Voriconazole is a new generation of triazole broad-spectrum antifungal drug approved for marketing in the United States in May 2002, developed by Pfizer. Based on the chemical structure of fluconazole, the drug is made by replacing the triazole ring in fluconazole with a fluoropyrimidinyl group and adding an a-methyl group. Voriconazole has two preparations, oral and injectable, with a broad antibacterial spectrum. It is the first choice for invasive aspergillosis, invasive candidiasis, and apical scedosporosis and fusarium disease that cannot tolerate other drugs or are ineffective for other drugs. drug. Based on a large amount of clinical evidence, voriconazole was listed as the drug of choice for invasive aspergillosis in the guidelines for the treatment of aspergillosis formulated by the Infectious Diseases Society of America (IDSA) in 2008. Voriconazole has good pharmacokinetic characteristics, showing nonlinear pharmacokinetic characteristics, bioavailability as high as 96%, and wide distribution in the body. It can pass through the blood-cerebrospinal fluid barrier and has high tissue concentration. Voriconazole is metabolized by liver enzyme system, and the metabolite is excreted through bile urine.
Voriconazole is mainly metabolized in the liver through cytochrome P450 (CYP450) isoenzymes CYP2C19, CYP2C9, and CYP3A4. CYP2C19 is the main metabolic enzyme, which has genetic polymorphisms, resulting in significant differences in the metabolism of voriconazole in different races and genotypes. Differences in plasma concentration after taking the same dose of voriconazole may lead to increased adverse reactions or poor curative effect. In addition, voriconazole is prone to drug interactions through P450 metabolism, which may result in the change in the plasma concentration of voriconazole due to the combination of drugs. For this reason, foreign scholars suggest that when conditions permit, the therapeutic drug monitoring of voriconazole should be carried out in a timely manner, the dosing regimen should be adjusted, and individualized dosing should be carried out, to improve the safety and efficacy of voriconazole application.
The purpose of this study is to establish a rapid, sensitive and accurate analytical method for the determination of voriconazole plasma concentration, to provide conditions for clinical therapeutic drug monitoring (TDM) of this drug, and to test voriconazole plasma concentration of 5 cases of invasive fungal disease patients with this method, to explore the application of this method in TDM.
01 Method
Schizophrenia is a chronic and severe mental illness. According to the data of the World Health Organization, more than 23 million people worldwide are troubled by schizophrenia, which mainly occurs in early adulthood, and its lifetime prevalence rate is about 3.8‰-8.4‰. Regional research data on schizophrenia in recent 10 years show that the lifetime prevalence rate of schizophrenia in Hainan Province was as high as 13.7‰ in 2014.
02 Results
Method specificity
The chromatogram measured under the selected chromatographic conditions is shown in Fig. 1, and the retention times of voriconazole and internal standard are shown in Fig. 1 as 4.1min and 8.3min respectively. The peak shape of the chromatographic peaks is good, the separation is complete, and the blank plasma has no interference from the peaks of voriconazole and the internal standard.
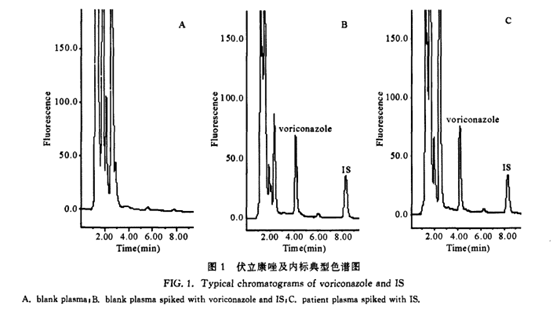
Plasma solutions containing 10 mg/L of fluconazole, caspofungin, metronidazole, cefoperazone, cefuroxime, roxithromycin, and levofloxacin were prepared respectively, and treated with the same treatment method as voriconazole blood samples before injection. All samples had no interference peaks at voriconazole and internal standard.
Standard Curve and Linear Range
There is a good linear relationship between the voriconazole plasma concentration and the fluorescence detection peak area ratio of the internal standard. Through linear regression, the standard curve for measuring voriconazole in the plasma is Y=0.221X-0.00636 (Y: the peak area ratio of voriconazole and the internal standard; X: concentration of voriconazole), regression coefficient r2=0.9993. The linear range of the standard curve is 0.1-50mg/L.
Extraction recovery rate, accuracy and repeatability
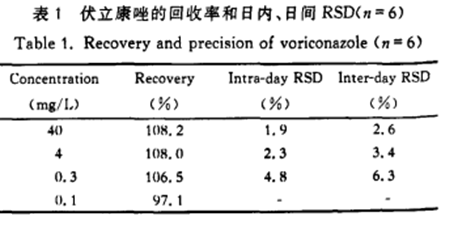
Calculate Voriconazole (abbreviated Vori) and internal standard (abbreviated IS) peak area ratio in 4 concentrations (0.1mg/L, 0.3mg/L, 4mg/L and 40mg/L) voriconazole buffer solution sample and plasma solution sample, and the average extraction recovery rate of 6 times of determination of each concentration was 107.6%±1.9%. Low, medium and high concentration plasma quality control samples were processed 6 times on the same day, the relative standard deviation (RSD) of the Et internal determination results was 1.9%-4.8oA, and the recovery rate of the intra-day method was 93.3oA-103.7%. Plasma quality control samples with low, medium and high concentrations were processed 6 days, the relative standard deviation (RSD) of the inter-day determination results was 2.6%-6.3%, and the recovery rate of the inter-day method was 87.7%-105.3%. The results of extraction recovery rate, intra-day and inter-day accuracy are shown in Table 1.
Stability study
(1) Placement stability of stock solution
Compared with the peak area of the freshly prepared stock solution of the same concentration, the relative recovery rate of the voriconazole stock solution after placing at -80°C for 3 months was 88.8%, and it dropped rapidly to 71.2% after placing for 4 months.
(2) Placement stability of plasma samples
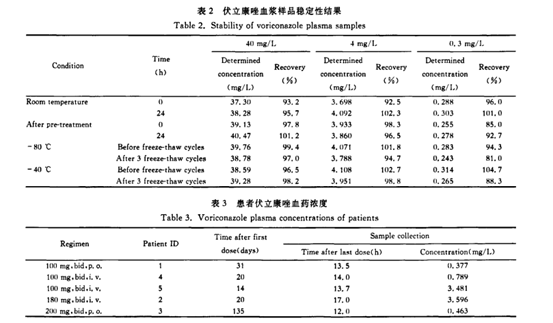
Plasma quality control samples with three concentrations of low, medium and high were placed at room temperature for 0, 3, 6, 9, 12 and 24 hours and then processed and analyzed respectively. The placement stability of voriconazole plasma samples at room temperature was investigated. The recovery rate of each concentration after placing at room temperature for 24 hours was 95.7%-102.3%, see Table 2. Plasma quality control samples with three concentrations of low, medium and high concentrations were placed in the autosampler for 0, 3, 6, 9, 12 and 24 hours after processing for sample analysis, and the stability of voriconazole plasma samples after processing was investigated. After the sample is processed, the recovery rate of the measured concentration after placing for 24 hours is 92.7%-101.2%, see Table 2.
(3) Freeze-thaw stability of plasma samples
Plasma quality control samples with three concentrations of low, medium and high were frozen at -80°C or -40°C, and then thawed at room temperature. Repeated freezing and thawing 3 times in this way, the stability of the sample after freezing was investigated. The sample recovery rate after 3 freeze-thaws at -80°C was 81.0%—97.0%, and the sample recovery rate after 3 freeze-thaws at -40°C was 88.3% to 98.8%, see the table 2.
Determination of voriconazole concentration in patient plasma
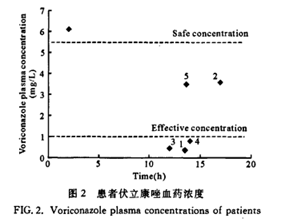
Five patients with pulmonary invasive fungal infection, 3 males and 2 females, with an average age of (55±17) years, an average height of (169±6) cm, and an average weight of (58±15) kg, and the pathogenic fungi are mainly Aspergillus, Cryptococcus and Candida. Five patients received voriconazole 100-180 mg orally or intravenously, twice a day. After continuous administration of 14 -31 days. The measured trough concentration before administration (blood collection 13.5-17 hours after the last administration) ranged from 0.377 to 3.596 mg/L, see Table 3.
04
Conclusion
The established method is accurate, sensitive, specific and rapid, and is suitable for the monitoring of therapeutic drug concentration and pharmacokinetic research of voriconazole. The plasma concentration of 5 patients was measured by this method, and the results were very different, suggesting that monitoring of voriconazole therapeutic drug concentration should be carried out, and individualized medication is necessary.

Fast
Testing speed up to 200 test/hour, the first result time ≤ 15 min
High capacity
25 reagent locations, 60 samples locations.
Small size
175kg, 100(length)*67(depth)*64(height)cm
Easy operation
batch testing mode, random testing mode, emergency mode

References:
Zheng Yunyan. Li Xiaodong. Clinical analysis of 80 cases of hospital deep fungal infection [J]. Chinese Journal of Leprosy and Skin Diseases, 2007, 23(12): 1 108-1 109.
Cao Yongbing, Zhang Lei, Wang Yan, etc. Voriconazole and its clinical application[J]. Chinese Journal of New Drugs and Clinics, 2005. 24(4): 330-332.
Zhu Jing. Pharmacological properties and clinical application of voriconazole]. Tianjin Pharmaceutical, 2007, 19(4): 48-50.
Jiang Zhengli. Zhu Ping, Lin Jianqun. New progress in research on triazole antifungal agents[J]. Chinese pharmaceutical industry. 2008, 17(4): 19-20.
Shen Hairong. Li Zhongdong. Zhong Mingkang. New antifungal drug voriconazole [J3. Chinese Journal of New Drugs and Clinics, 2004, 23(5): 308-310.
This article is excerpted from Chinese Journal of Infection and Chemotherapy, Volume 9, Issue 2, March 20, 2009. Wu Xiaojie, Dong Xiaoyi, Chen Yijian, Zhang Jing, Shi Yaoguo, Wu Jufang, Zhang Yingyuan.