This study was a collection of 61 adult patients with multidrug-resistant gram-positive bacterial infections from 2015-2017 in Udine, Italy. Patients received more than 10 days of linezolid therapy. Patients' linezolid regimen was a standard 600 mg dose every 12 hours with possible adjuncts to other combination therapies. Platelet count, hemoglobin concentration, C-reactive protein, and serum creatinine are monitored during the course of treatment, and Cmin is measured by TDM with the goal of controlling linezolid Cmin to 2 - 8 mg/L. In this study, patients will be divided into groups according to linezolid exposure as follows: adequately stabilized (Group A), overexposed (Group B), and underexposed (Group C). Group B is divided into subgroup B1, which can reach the target range by TDM stabilization, and subgroup B2, which cannot; Group C is divided into group C1, which can reach the target range, and group C2, which cannot reach the target range.
At the end of the trial, the final enrollment of 61 patients showed 28 (45.9%) in group A, 33 (54.1%) in group B (29 in subgroup B1 and 4 in subgroup B2), and 0 in group C. The final enrollment of 61 patients in group B was in group C, and the final enrollment of 61 patients in group B was in group C. The patients were predominantly male, with a median age of 62 years, and the main type of infection was bone and joint infections, with methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-resistant coagulase-negative staphylococci (methicillin-resistant CoNS) being the most common etiologic agents. Although the initial treatment was performed using the same strategy, there was a significant difference in the treatment regimen of group A versus group B1 in the case of TDM intervention (17.9 vs 12.8 mg/kg/day; p<0.001), and there was also a difference in treatment between group A and group B2 (17.9 vs 13.3 mg/kg/day, p=0.002). There was also a difference in Cmin between group A and groups B1 or B2 (3.8 VS 5.1 mg/L and 3.8 VS 8.3 mg/L, respectively, p<0.001 ).
Incidence of thrombocytopenia: The overall incidence of thrombocytopenia was 14.8% (9/61), which was significantly lower in subgroup A (10.7%, 3/28) and subgroup B1 (10.3%, 3/29) than in subgroup B2 (75.0%, 3/4). Patients in subgroup B1 who developed thrombocytopenia gradually recovered without stopping the medication until the end of the treatment program under the guidance of TDM.
The final patient groupings and the occurrence of side effects are shown in Figure 1. The parameter characteristics of patients who developed.
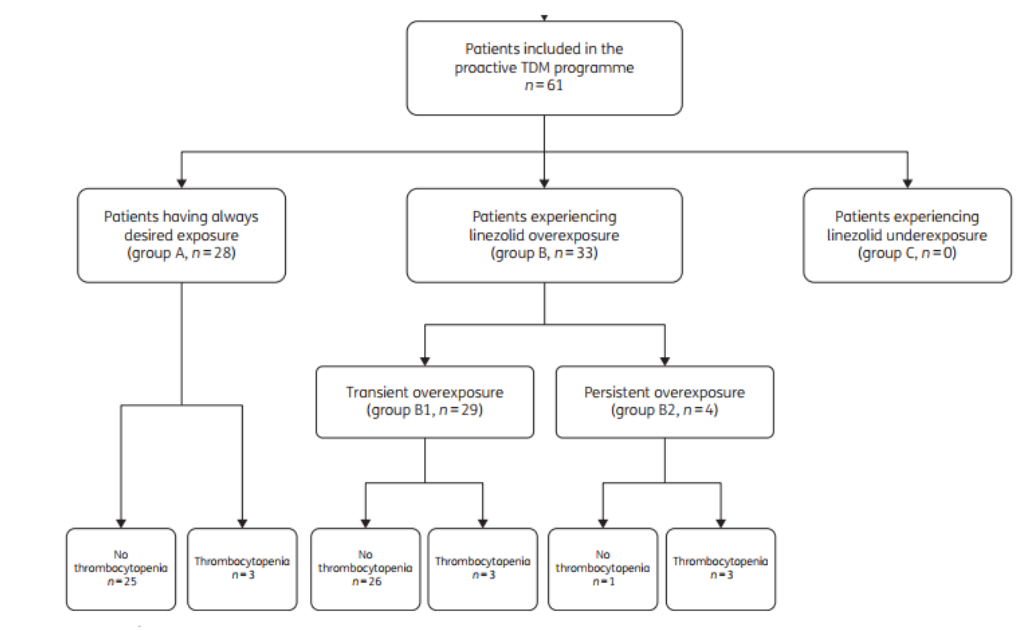
Figure 1. Study flow chart
Table 1. Characteristics of those patients who experienced thrombocytopenia during linezolid treatment (n=9)
Univariate and multivariate linear regression analyses showed that the probability of thrombocytopenia independently was significantly associated with linezolid trough concentration Cmin and limiting platelet technique (as shown in Table 2).
Table 2. Univariate and multivariate regression analysis of variables associated with the occurrence of thrombocytopenia (n=61 patients)
This study investigated the role of aggressive TDM in optimizing linezolid exposure and preventing and restoring dose-dependent thrombocytopenia. Linezolid-induced thrombocytopenia is the main adverse effect and may lead to treatment interruption. The prevalence of thrombocytopenia was low in early clinical trials, but was higher in subsequent real-world studies in different patient populations treated with fixed doses (600 mg every 12 hours) for more than 10 days [3 ].
In the present study, aggressive TDM focused on controlling linezolid Cmin to 2-8 mg/L was beneficial in preventing and recovering from thrombocytopenia, even with long-term treatment.The incidence of thrombocytopenia in patients in groups A and B1 subgroups was much lower than that in previous non-interventional studies. Although there is a Spanish prospective single-center study against the role of TDM, it was non-interventional and only a single TDM assessment was performed, unlike the present study.
Multivariate regression analysis showed that median linezolid Cmin and baseline platelet count were variables independently associated with the risk of thrombocytopenia. Previous studies have shown an association between treatment duration and risk of thrombocytopenia [4], but this association was not found in the present study, which is consistent with the results of another interventional study, which found that lowering the dose of linezolid in high-risk patients prevented adverse effects. In addition, this study found that stabilizing linezolid Cmin in the target range after a transient drug overdose helped patients recover from thrombocytopenia without stopping the drug.
This study also has limitations, such as the lack of a control group, the small sample size, the inability to clarify the relationship between linezolid exposure and clinical efficacy, and the inability to rule out the effect of coadministration as a confounding factor on thrombocytopenia. However, the prospective study design has advantages in assessing the safety of long-term treatment.
Overall, this study demonstrates that aggressive linezolid TDM is beneficial in preventing and recovering from dose-dependent thrombocytopenia, makes the management of long-term therapy safer and more feasible, and provides preliminary data for subsequent larger prospective studies.

Reference1. Pea, F., et al., Therapeutic drug monitoring may improve safety outcomes of long-term treatment with linezolid in adult patients. J Antimicrob Chemother, 2012. 67(8): p. 2034-42.
2. Pea, F., P.G. Cojutti, and M. Baraldo, A 10-Year Experience of Therapeutic Drug Monitoring (TDM) of Linezolid in a Hospital-wide Population of Patients Receiving Conventional Dosing: Is there Enough Evidence for Suggesting TDM in the Majority of Patients? Basic Clin Pharmacol Toxicol, 2017. 121(4): p. 303-308.
3. Grau, S., et al., Linezolid: low pre-treatment platelet values could increase the risk of thrombocytopenia. J Antimicrob Chemother, 2005. 56(2): p. 440-1.
4. Kim, H.S., et al., Linezolid-induced thrombocytopenia increases mortality risk in intensive care unit patients, a 10 year retrospective study. J Clin Pharm Ther, 2019. 44(1): p. 84-90.