In order to identify the reasons for the fluctuation in voriconazole concentration in the later data analysis, the enrolled subjects were all inpatients of this hospital and their first dose had to be administered in the hospital. In addition to the trough concentrations of the drugs, several data were collected, including the reason for administration, hepatic and renal function status, and concomitant use of other interacting drugs.
Based on the above criteria, a total of 56 patients were enrolled in this study. Of the initiate voriconazole trough concentration test, 41 (73.2%) reached the therapeutic range (1-5 mg/L). Of these, 30 (73.2%) maintained concentrations within the therapeutic range throughout treatment; 10 (24.4%) were out of range in level 2 (7 were ultrahigh and 3 were below the effective range), and 1 (2.4%) was out of range (low) in level 3. Of the 15 patients who were out of range in the initial assay, 11 (73.3%) were supratherapeutic, and 4 ( 26.7%) had subtherapeutic. In addition, 8 of these 15 patients had dose-adjusted blood concentrations restored to the therapeutic range, while 7 discontinued voriconazole treatment because of adverse effects or other reasons (as shown in Figure I).
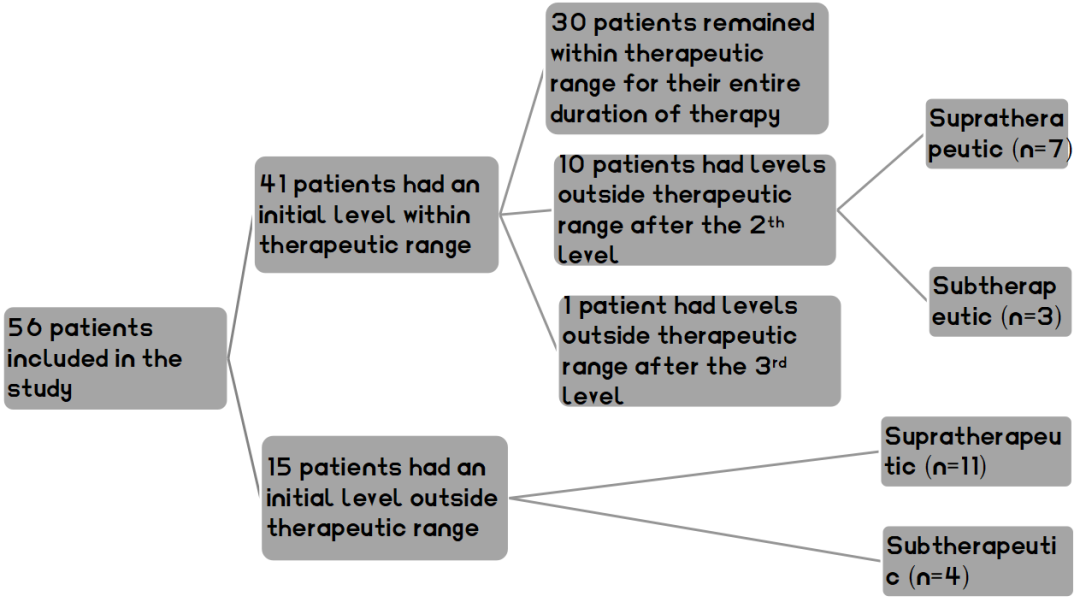
Figure1 Flow diagram for voriconazole therapeutic drug monitoring
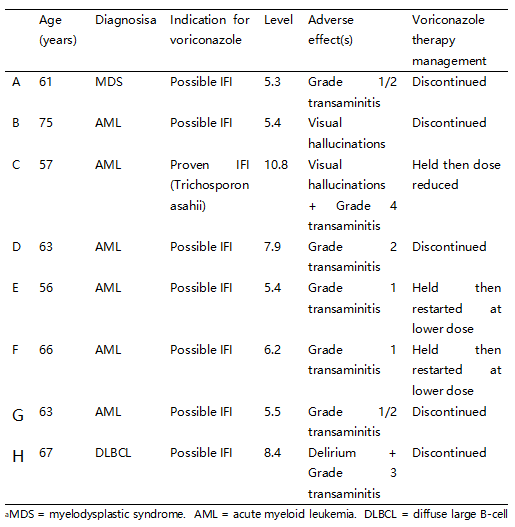
In the duration of treatment, 23 (41.1%) experienced adverse effects, and of these, 8 (34.8%) were found to be supratherapeutic. Table 1 describes in detail the diagnosis, infection and subsequent treatment of these 8 patients. 19 patients experienced transaminitis, 3 patients experienced both transaminitis and neurotoxicity, 1 patient experienced photopsia. For transaminitis specifically, there were 28 distinct episodes in total with 12 (42.9%) instances of Grade 1 transaminitis; 12 (42.9%) of Grade 2; 3 (10.7%) of Grade 3; and 1 (3.5%) of Grade 4. Of the 23 patients who experienced adverse effects, 5 (21.7%) discontinued voriconazole therapy.
39 patients were receiving at least one of the interacting drugs that may interference voriconazole metabolism. These drugs included the proton pump inhibitors pantoprazole and fluoxetine (which can theoretically increase voriconazole levels), and the CYP2C19 inducer letermovir (which can decrease voriconazole level). Thirty-four of them received pantoprazole during treatment. 25 of these 34 had an initial level within therapeutic range, 6 had a supratherapeutic initial level, 3 had a subtherapeutic initial level, and 18 patients experienced adverse effects (4 of them were supratherapeutic). 1 patient took letermovir but had initial level within therapeutic range and did not experience adverse effects. 2 patients received both pantoprazole and letermovir with level within therapeutic range and no adverse effects. However, 1 patient received both pantoprazole and fluoxetine and was supratherapeutic, but did not experience adverse effects.
Table 1. Patients who experienced adverse effects with supratherapeutic levels (n = 8).