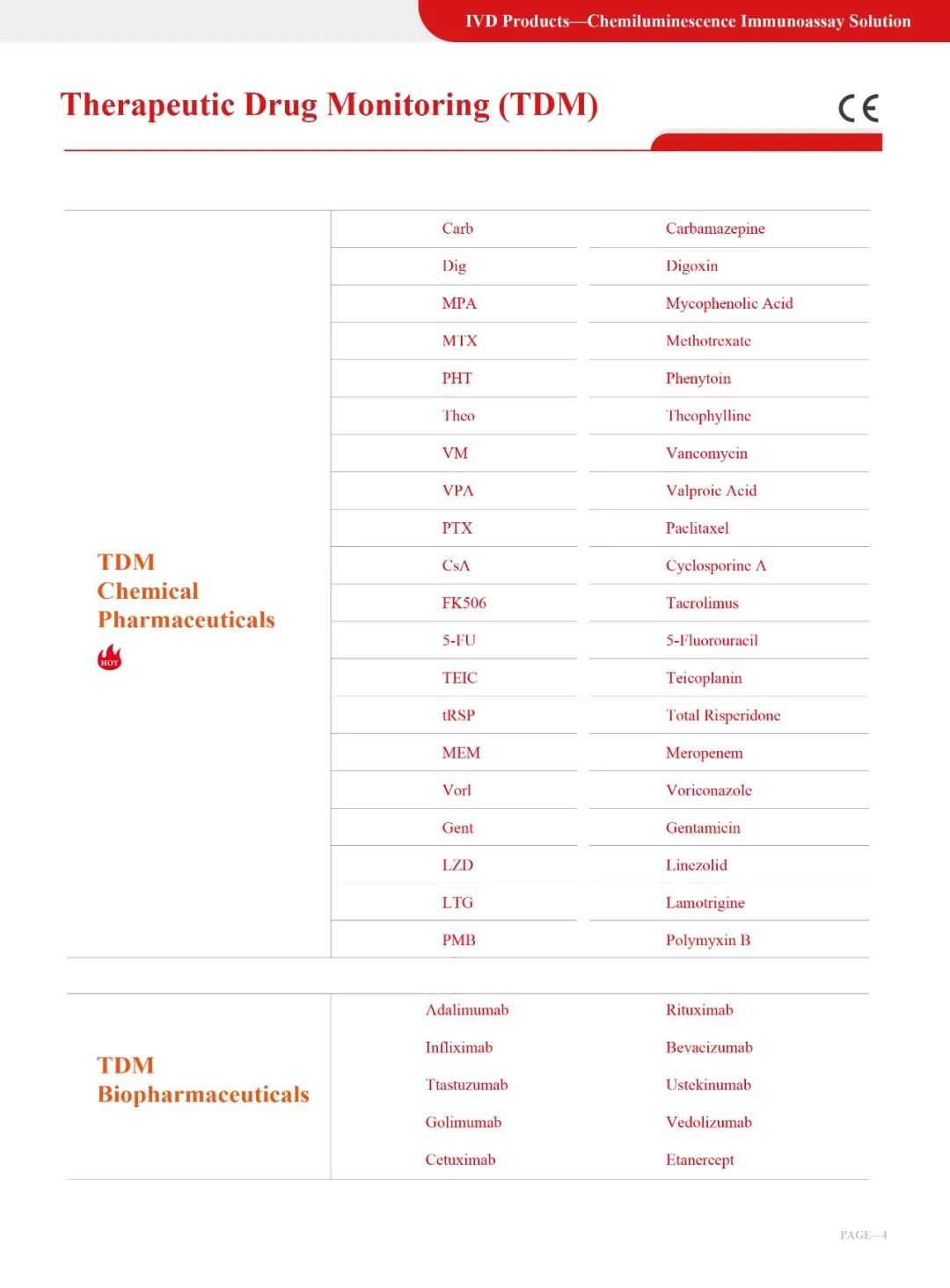
Current status of clinical application of risperidone
Risperidone is a classic psychotropic drug used to treat schizophrenia and bipolar mania. It has been in clinical use as early as 1993 and is still the most prescribed antipsychotic drug in the world. Risperidone is also used to treat manic episodes in people with schizophrenia aged 13 to 17 years and with bipolar disorder in people aged 10 to 17 years. And many countries even use it on children as young as 5 years old with mental retardation or autism. Although a large number of clinical trials have verified the effectiveness of risperidone, the safety of risperidone still deserves attention due to its side effects. Especially among adolescents, it can easily cause a variety of side effects, including weight gain, sedation, etc. In addition, extrapyramidal symptoms, hyperprolactinemia, and impaired glucose tolerance are also common [1]. There is still a lack of research on the dose dependence and ideal dose range of risperidone side effects in children and adolescents. It is necessary to increase awareness of the safe use of risperidone in children and adolescents.
Using TDM to study the blood concentration pattern of risperidone in children and adolescents
To evaluate the relationship between daily dose and serum concentration of risperidone in children and adolescents receiving risperidone for schizophrenia psychosis, this study used data from routine TDM to analyze and explore whether the recommended concentration range of the active moiety in the blood of adults also applies to children and adolescents with schizophrenia. In addition, the study also analyzed the impact of different patients and different treatments on serum concentrations.
The study collected blood samples from 64 patients aged 11-18 years at five medical centers in Vienna, Germany and Austria. The concentrations of risperidone and its active metabolite 9-OH-RIS were detected and analyzed using routine TDM technology in these blood samples.
Effect of risperidone dose and other covariates on RIS/9-OH-RIS (RISam) concentrations
Among the 64 patients included (11-18 years old), a positive correlation was found between the daily dose and the final blood concentration of active ingredient (RISam) (rs = 0.49, p = 0.001), which is consistent with the results of previous studies in adult patients. In children and adolescent patients, body weight, gender, body mass index, etc. have no correlation with the concentration of active ingredients in the blood. When analyzing whether combined use of drugs affects the plasma concentration of risperidone, it can be seen that although the combined use of CYP2D6 inhibitors has no effect on the total concentration of active ingredients in the patient's blood, the ratio of parent RIS to metabolite 9-OH-RIS was significantly changed. And the study by Fekete et al also showed that compared with adult patients, the 9-OH-RIS/RIS ratio in children and adolescents is lower, which may be due to the higher renal clearance rate of 9-OH-RIS in children and adolescents [2]. From the analysis of the relationship between blood drug concentration and side effects, the RISam concentration in patients with extrapyramidal symptoms (EPS) was on average higher than that in asymptomatic patients (p = 0.05).
Therefore, to avoid EPS, the recommended upper therapeutic range of RISam is 33 ng/ml. Comparing previous adult data, the initial treatment range for minors is also significantly lower than the recommended range for adults (9-33 ng/ml vs 20-60 ng/ml). These preliminary data may help define the therapeutic window for risperidone in the treatment of schizophrenia disorders in children and adolescents. The use of TDM is recommended in these susceptible populations to prevent concentration-related adverse drug reactions.
References:
1. Solmi, M., et al., Safety of 80 antidepressants, antipsychotics, anti-attention-deficit/hyperactivity medications and mood stabilizers in children and adolescents with psychiatric disorders: a large scale systematic meta-review of 78 adverse effects. World Psychiatry, 2020. 19(2): p. 214-232.
2. Fekete, S., et al., Dose-Corrected Serum Concentrations and Metabolite to Parent Compound Ratios of Venlafaxine and Risperidone from Childhood to Old Age. Pharmacopsychiatry, 2021. 54(3): p. 117-125.
Diagreat Therapeutic Drug Monitoring (TDM) program