Pain Points in Cardiac Marker Testing
Pain Point 1: Interference with cTn
The structural properties of cTn can cause potential interferences that can lead to false positive or false negative results. For example, enzymatic cleavage sites, phosphorylation and glycosylation sites, autoantibody binding sites and so on. To address this pain point, Diagreat Biotech R&D process technology has been upgraded again, with the breakthrough of using a proprietary technology to obtain the (Fab)2 fragment of the antibody and ligating alkaline phosphatase directionally to the neck loop region of the antibody.
✓ Fc-free fragment: extremely low HAMA, complement interference with cTnT Cap03 antibody.
✓ Highly homogeneous: SEC-HPLC single peaks 95%+, brings reagent process homogeneity, excellent CV, very low lot-to-lot variation.
✓ Directed coupling: well preserved Fab activity, better reactivity.
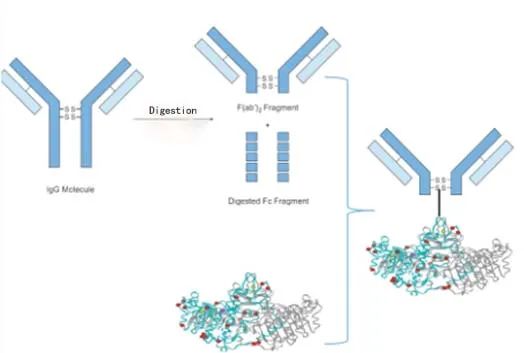
cTnTDet Fab-ALP preparation process
Pain point 2: Difficult to meet patients' needs for different choices of myocardial testing
For clinical myocardial testing, some choose hs-cTnT or hs-cTn I, and due to the restriction of hs-cTnT patent, it is rare to develop and market hs-cTnT and hs-cTn I at the same time. With the breakthrough of the localization of hs-cTnT, Diagreat Biotech breakthroughs to realize the technical upgrading of hs-cTnT and hs-cTn I, which can satisfy the needs of different patients for high-sensitivity troponin I/T testing! Diagreat Biotech has a more complete myocardial testing catalog and better production process quality, which greatly meets the multi-dimensional patient testing needs.