Digoxin is a form of digitalis commonly used in clinical settings. It is a moderately potent cardiac glycoside that promotes positive inotropic action, slows down heart rate, and inhibits heart conduction. It is mainly used to treat congestive heart failure, atrial fibrillation, paroxysmal tachycardia, and premature ventricular contraction-related disease.
Digoxin has a narrow therapeutic window, with the therapeutic dose being close to 60% of the toxic dose. The bioavailability of Digoxin and the sensitivity of patients to Digoxin are also highly individualized. Varying levels of toxic reaction to Digoxin is commonly observed, with cardiotoxicity being the most severe as it could lead to various types of arrhythmias. These could be fatal in the worse case. At the same time, clinical signs of Digoxin therapy effect are very similar to the cardiotoxic signs, masking the cardiotoxic conditions and delaying the treatment to reverse Digoxin overdose.
Within Digitalis-type drugs, Digoxin is excreted relatively fast, and a relatively small amount accumulates within the body. The absorption of oral form Digoxin is incomplete and highly irregular. The Chinese Guidelines for the Diagnosis and Treatment of Heart Failure 2018 published by the Chinese Society of Cardiology Heart Failure Group, et al suggested strict monitoring for Digoxin poisoning and other adverse reactions and maintaining the patient drug concentration to between 0.5-0.9 ng/mL. The therapeutic serum concentration of Digoxin has been universally recognized as between 0.8-2.0 ng/mL. The Pharmacokinetics of Digoxin is also highly varied between individuals. Internal and external factors within patients may also affect Digoxin pharmacokinetics. Therefore, Therapeutic Drug Monitoring is very important for patients treated with Digoxin in terms of clinical safety and individualized drug regimen formulation.
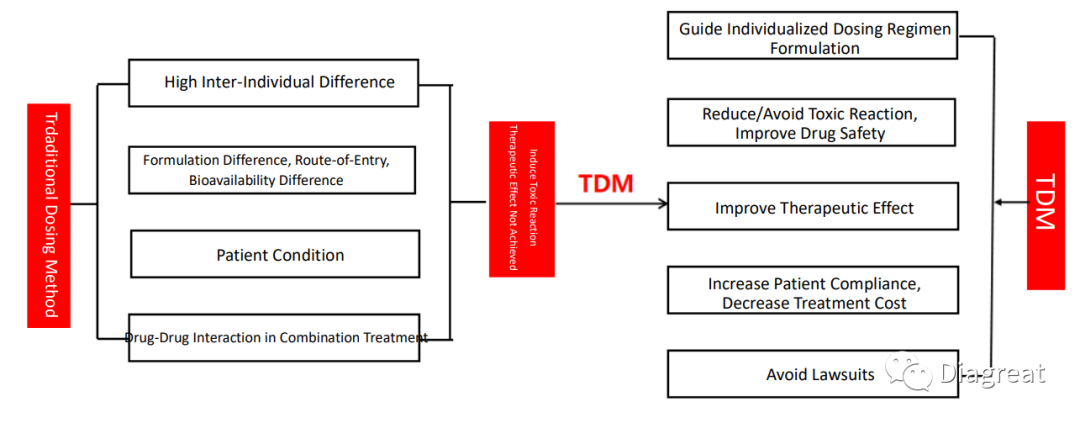
Introduce TDM to Realize Individualized Treatment
Therapeutic Drug Monitoring (TDM): Conceptualized on pharmacokinetics theory, use sensitive and reliable analytical techniques, detect drug concentration within patient blood or other body fluid, analyze the relationship between drug concentration and therapeutic effect&toxic response, then design and adjust the drug regimen based on detection. Diagreat develops TDM technology to meet the clinical demand for easy to operate, fast detection speed, reliable, economical, so more patients may benefit.