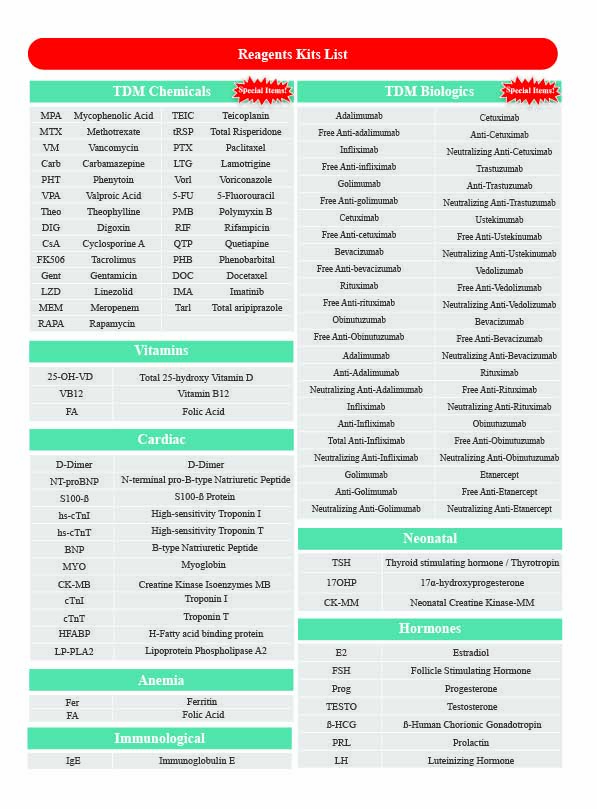
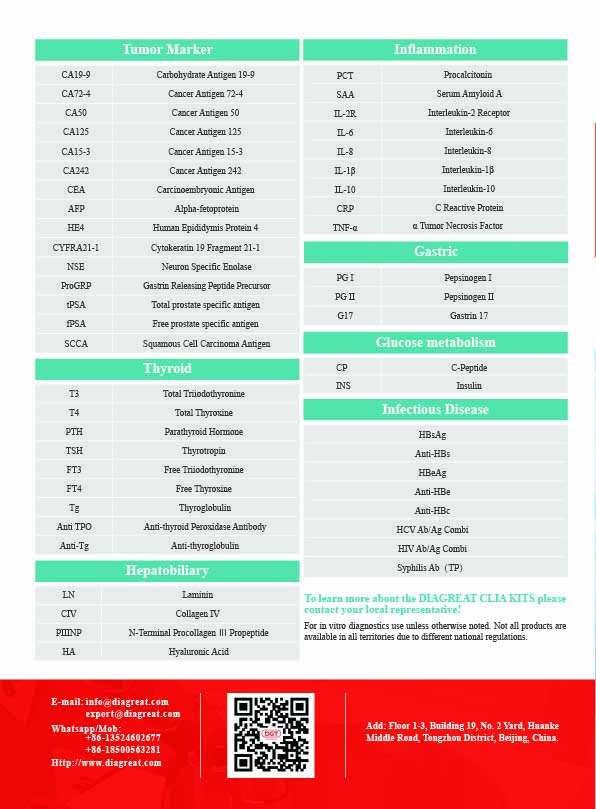
Adalimumab determination kit is an in vitro diagnostic test for the quantitative detection of free adalimumab in human serum or plasma specimen.
Adalimumab is approved for treatment of rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, pediatric and adult Crohn disease, ulcerative colitis, etc.
Testing for adalimumab concentration and presence of anti-adalimumab antibodies is helpful to adjust therapeutic strategies for patients starting therapy (proactive monitoring), and to adjust dosing or treatment strategy when partial response or loss of response to therapy is observed, manifested as recurrence of symptoms.