In 2020, Coronavirus (COVID-19) is raging around the world. In February, Diagreat successfully developed the Coronavirus detection kit, 2019-nCoV IgG Antibody Determination Kit, which is based on the Fluroescence Immunoassay.
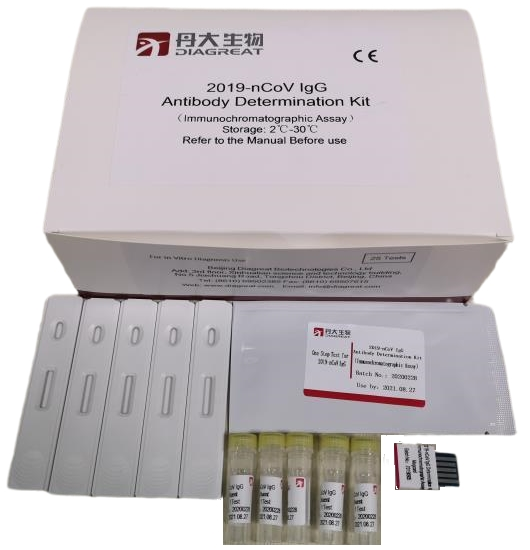
Product information
Product Name | Methodology | Size | Storage and Validity | Specimen |
2019-nCoV IgG Antibody Determination Kit | Fluorescence immunochromatography | 25 Tests/box | Stored at 2-30℃ within 18 months | Whole blood Serum Plasma |
2019-nCoV IgM Antibody Determination Kit
|
Intended use
The 2019-nCoV IgM Determination Kit is used for qualitative determination of IgM&IgG antibodies in human serum, plasma and whole blood. The kit is offered to clinical laboratories and healthcare workers for point-of-care testing, and not for at home testing, in compliance with Section IV.D. of the FDA's Policy for COVID-19 Diagnostic test.
Clinical Application
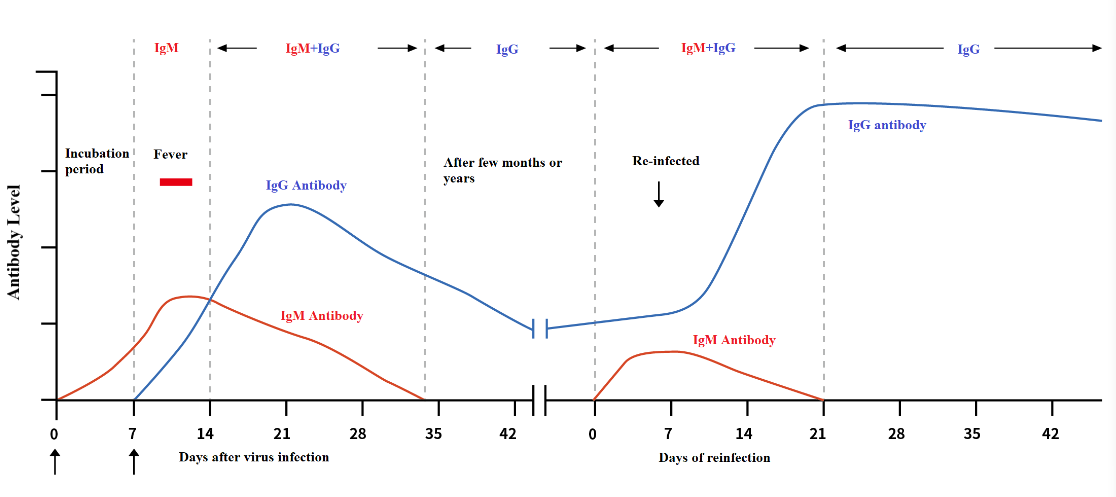
In human infected with a novel coronavirus (COVID-19), the body's immune system produces an immune response to the virus, producing specific antibodies. The determination of relevant antibodies can be used to screen for infection with novel coronavirus early.
According to Diagnosis and Treatment of COVID-19 (7th Edition) issued by National Health Commission of the People's Republic of China, 2019-nCoV IgM can be detected about 3-5 days after the onset of COVID-19 and IgG titer of patient in recovery phase is 4 times or more comparing to patient in acute phase.
Advantages
Short test time: Get results in 15 mins.
Accurate Result: Fluorescence immunoassay, which is high sensitivity.
Operation: Easy for operators, no professional skill required.
Early detection: Early diagnosis and screening of suspected cases.
Reduce misdiagosis rate: Lower the misdiagnosis rate by nucleic acid.
POCT analyers for 2019-nCoV


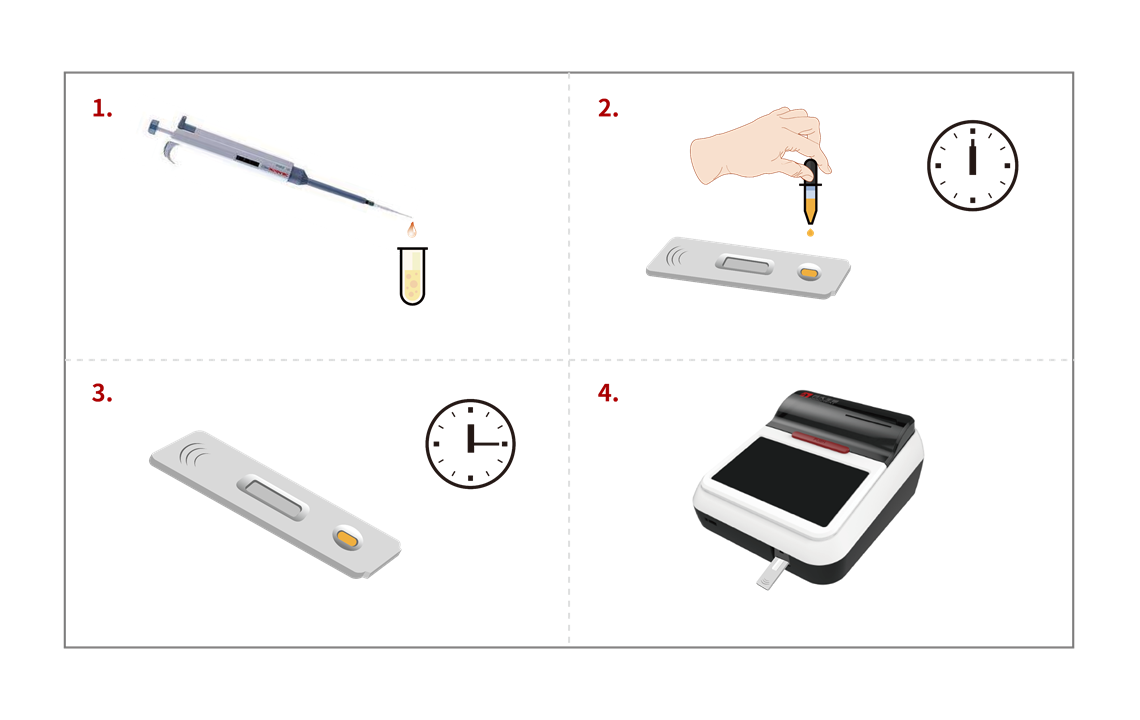
Procedure
Clinical Research
The 2019-nCoV IgG/IgM Antibody Determination Kit has been recommended as quick, simple and accurate detection method for the suspected cases, which supported by clinical research performed by specialists of clinical laboratory medicine from Shenzhen Hospital of Southern Medical University (Shenzhen, China) and Chinese PLA General Hospital (Beijing, China).
The research has been preprint at MedRxiv in 3, March, 2020,
(doi: https://doi.org/10.1101/2020.02.28.20029025)
Note:
· This test has not been reviewed by the FDA.
· Negative results do not rule out SARS-CoV-2 infection, particularly in those who have been in contact with the virus. Follow-up testing with a molecular diagnostic should be considered to rule out infection in these individuals.
· Results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection or to inform infection status.
· Positive results may be due to past or present infection with non-SARS-CoV-2 coronavirus strains, such as coronavirus HKU1, NL63, OC43, or 229E.
Not for the screening of donated blood.
Instruction for use
 6372142198788930846626315.pdf
6372142198788930846626315.pdf
Mexican Distributor:
Asiatic Connection
Contact in Mexico: Jorge Eduardo Valencia
Mexico Mobile/Whatsapp: 0052 55 8611 6009
Chinese mobile/whatsapp: 0086 15601917760
Email: jorval@asiatic-connection.com
Wechat: jorvalmex

Chile Distributor:
Asiatic Connection
Contact in Chile: Marcelo Fernandez
Chile Mobile/Whatsapp: +56 956726977
Chinese mobile/whatsapp:
Email: m.fernandez@asiaticchile.cl
Wechat: marcelofernandez