In 2020, Coronavirus (COVID-19) is raging around the world. In February, Diagreat successfully developed the Coronavirus Antigen kit, 2019-nCoV Antigen Rapid Test Kit, which is based on the Colloidal Gold Assay.
Intended use
The rapid test kit is used for qualitative determination of novel coronavirus (SARS-CcV-2) antigen in human nasal swab and saliva samples in vitro.. This kit is offered to clinical laboratories and healthcare workers for point-of-care testing, and not for at home testing, in compliance with Section IV.D. of the FDA’s Policy for COVID-19 Diagnostic Test.
Principle
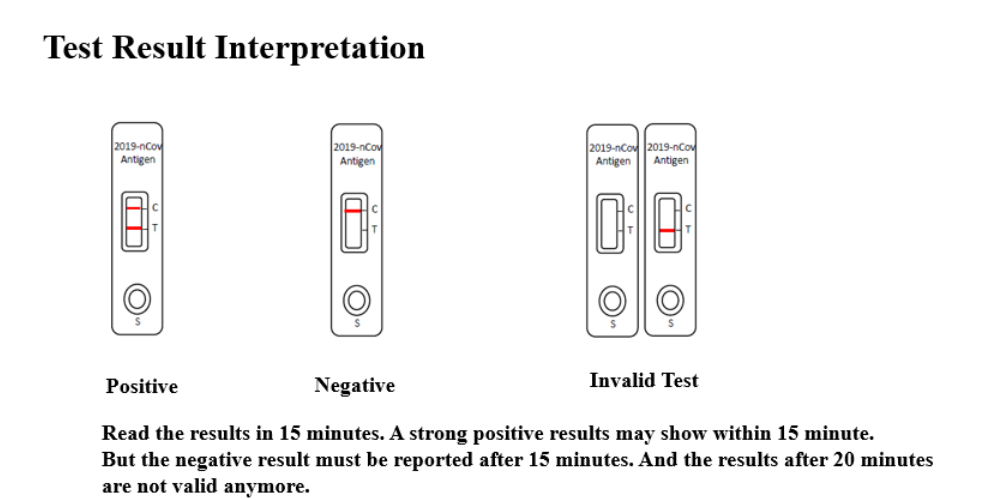
After an appropriate amount of sample is added to the detection well, the sample moves under the action of the capillary. The new coronavirus antigen in the sample will combine with colloidal gold-labeled new coronavirus N protein antibody to form a colloidal gold-antigen-antibody complex. The immune complex product is then chromatographed along the nitrocellulose membrane to the detection area (T), binds to the pre-coated N protein monoclonal antibody, and forms a purple line, indicating that the new coronavirus antigen is positive.
The quality control antibody-labeled colloidal gold particles are chromatographed to the quality control area (C) and combined with the pre-coated anti-quality control antibody to form a purple C line, indicating that the test is effective. If the QC line does not appear, the test result is invalid.
Applicable Department
Emergency Department
ICU
Pneumology Department
Cardio-Pulmonary Function Department
Manual
English
 6379341268078765392338176.pdf
6379341268078765392338176.pdf
Spanish
 6379341269364707171142213.pdf
6379341269364707171142213.pdf
German
 6379341270705333322547899.pdf
6379341270705333322547899.pdf
French
 6379341272763150106760937.pdf
6379341272763150106760937.pdf
Italian
 6379341276945970976815314.pdf
6379341276945970976815314.pdf
Estonian
 6379341283310048258138269.pdf
6379341283310048258138269.pdf
Portugues
 6379341286272551846787665.pdf
6379341286272551846787665.pdf
Clinical Data
 6375263086600395242507036.pdf
6375263086600395242507036.pdf
The latest clinic data report from Poland Centralne Laboratorium Kliniczne Uniwersyteckie Centrum Kliniczne (University Clinical Center)
 6376307035069620295495053.pdf
6376307035069620295495053.pdf